Chemistry at Envisioning Research Contests
Our department has done exceptionally well in the 2021 Envisioning Research contest. Take a look and enjoy the scope and depth of our research!
The Envisioning Research Contest is a collaborative effort by NC State’s Office of Research and Innovation, the Graduate School, the NC State University Libraries, the Office of Undergraduate Research and University Communications and Marketing. Envisioning Research is open to faculty, staff, graduate students, postdoctoral researchers and undergraduates.
2022
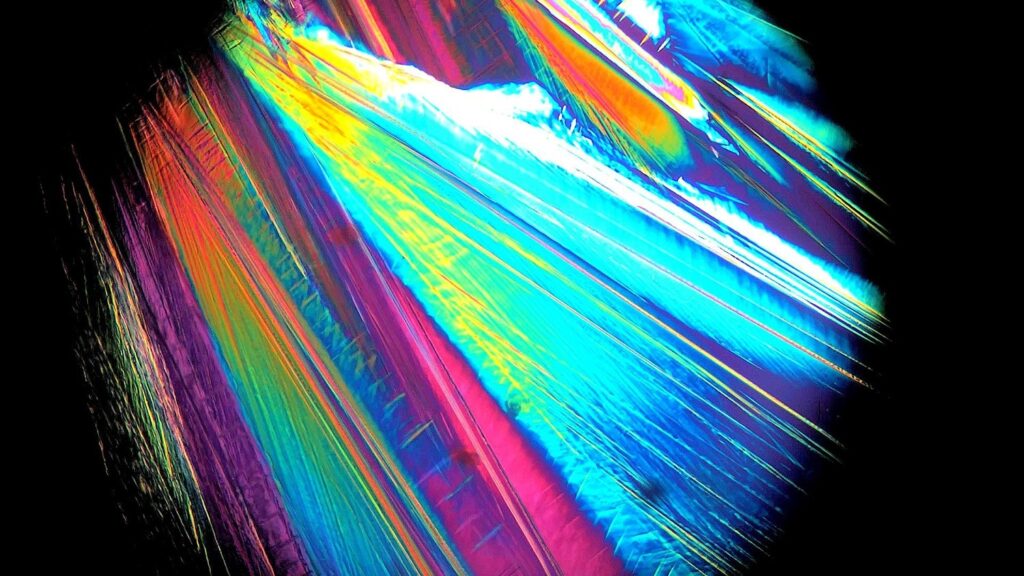
Polarized Optical Microscopy of Zinc Chloride from Solution
Undergraduate Student Microscopy, 1st Place, by Sydney Andersen
We are studying crystal growth from solutions with varying zinc chloride concentrations to better understand the mechanisms of crystal growth to develop an improved crystal growth model.
Synthesis of New Fluorescent Dyes
Graduate Student/Postdoc Video, 2nd Place, Christopher Brewer
To purify the compounds, it is easiest to work with them if they are dissolved in solution. However, some of the compounds have very low solubility. Instead of using massive volumes of solvent to dissolve the compounds, which would be wasteful and challenging to work with, a technique called Soxhlet extraction can be used. The upper portion of the apparatus contains the fluorescent dye and the bottom portion contains the solvent. The solvent is distilled from the bottom portion to the upper portion and some of the compounds will dissolve. Once filled to a certain height, the liquid will siphon down. Then the clean solvent is once again distilled to the upper portion and the cycle repeats until fully dissolved.
2021
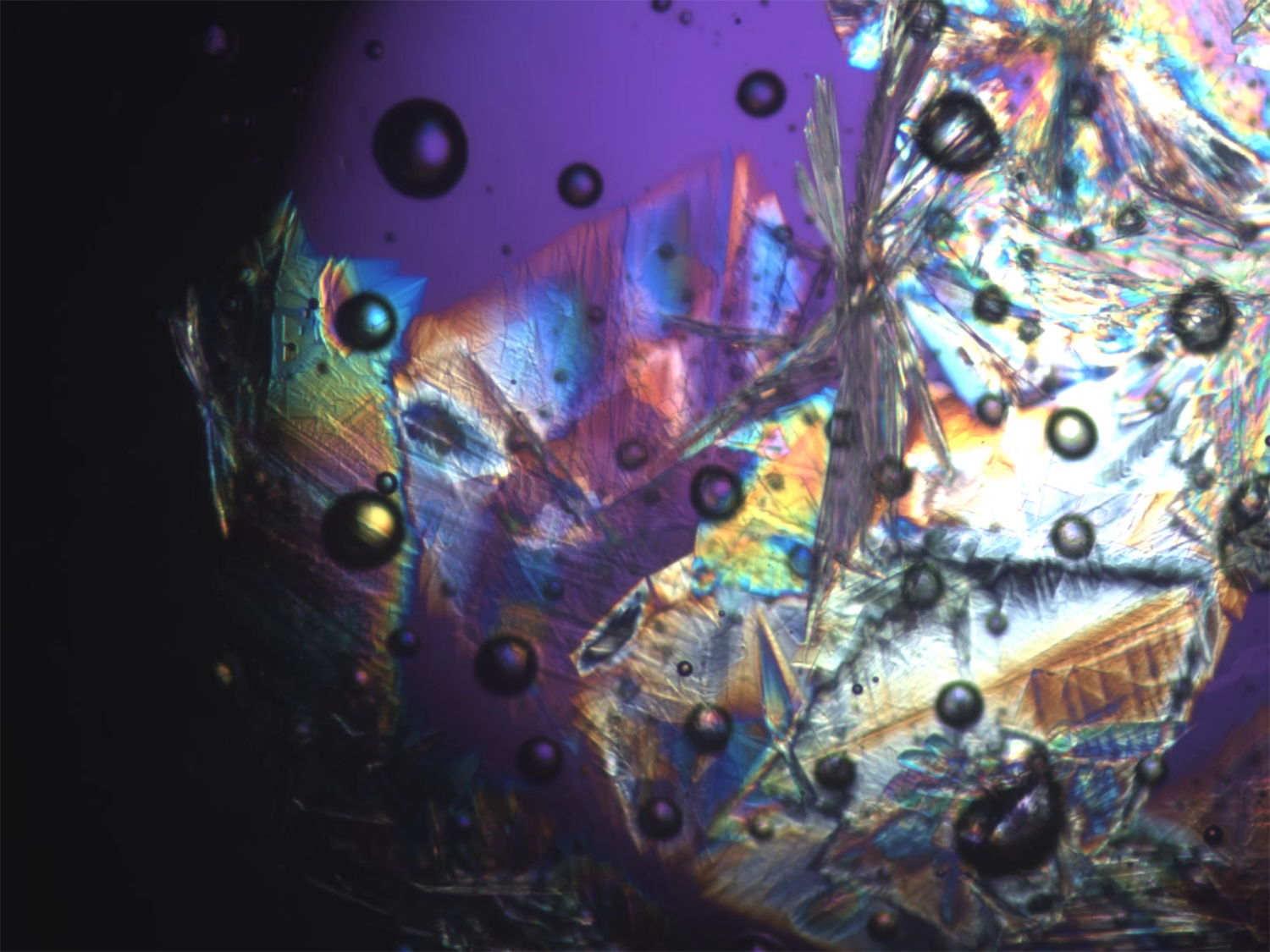
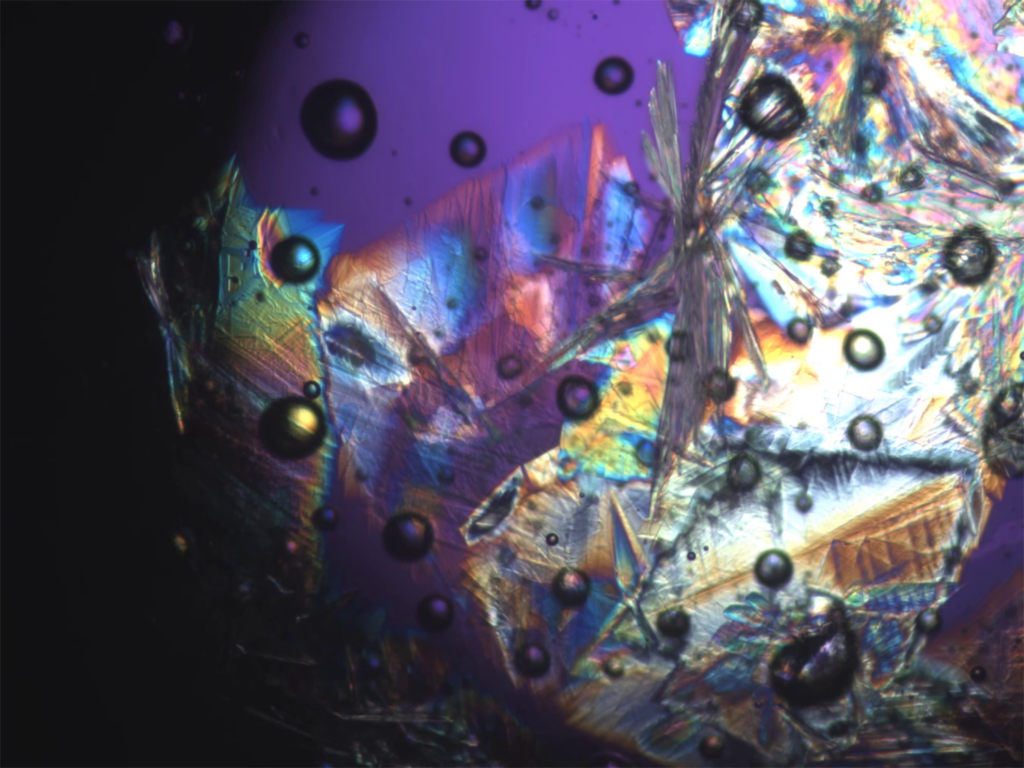
Microscopic Peacock Tail: Fanning Zinc Chloride Trihydrate Crystals
Graduate Student/Postdoc Microscopy, 2nd Place, by Shelby Boyd
By studying the crystallization rate of this ionic liquid at various temperatures, we can develop models of crystal nucleation and growth that are valid across a wider range of conditions than current models.
Photochemical Upconversion in Micellar Assemblies
Graduate Student/Postdoc Graphics, 1st Place, by Rémi Fayad
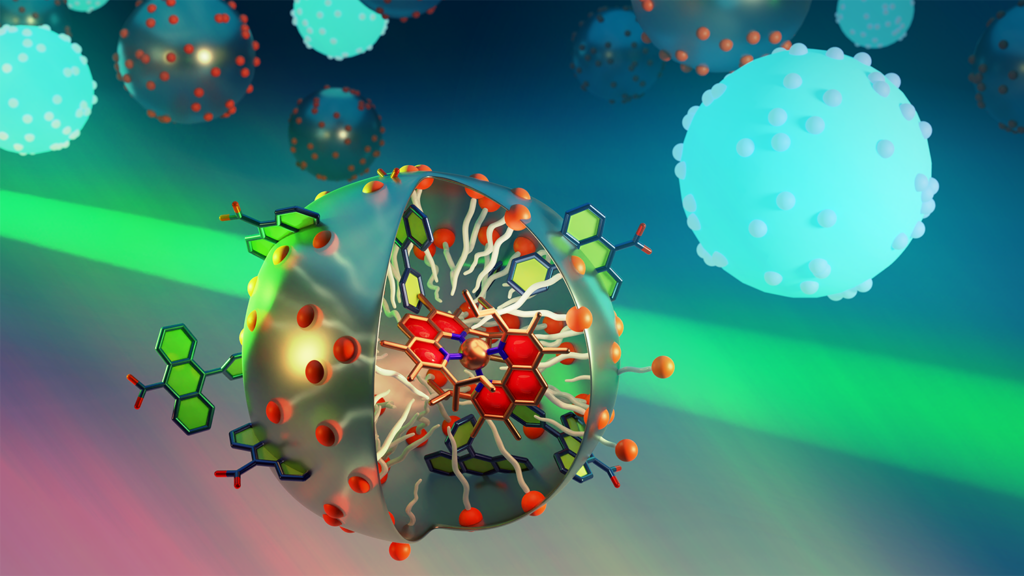
In this project, we used a light absorber based on earth-abundant elements confined in a micelle and an energy acceptor to generate a high energy photon (deep blue) from 2 lower energy photons (cyan blue) in water. This process, known as photochemical upconversion, was achieved by triplet-triplet annihilation of the energy acceptor excited states. While this process is very well studied in organic solvents, it proves to be challenging in water for multiple reasons. The micellar design (see image) provided a viable route to circumvent these challenges and to realize photochemical upconversion in neat water which can open the scope of studying many photoredox applications that operate under environmental-friendly conditions and using earth-abundant copper metal as the photosensitizer.
Authors: Rémi Fayad, Anh Thy Bui, Samuel G. Shepard, Felix N. Castellano
NC State in a Flask
Graduate Student/Postdoc Photography, Honorable Mention, by Alejandro Valdes and Aymee Alvarez
Phenyl pyruvic acid derivatives are very useful building blocks used for the synthesis of synoxazolidinones and pyrrolidinediones, among other many chemical compounds. In the Pierce Lab, we access phenyl pyruvic acids through hydrolysis of azlactones that form via Erlenmeyer reaction. In this picture, para-trifluoro phenyl pyruvic acid synthetized in gram scale yielded this deep red-colored solution. The immobile solvent surface allows to see NC State’s north campus on the horizon, visible through a lab window on the 8th floor of Dabney Hall.
2020
Colorful Solutions in Test Tubes
Graduate Student/Postdoc Photography, Honorable Mention, by
Sara Sheykhi.
I collected these colorful solutions in test tubes as they flowed from a silica gel column, which is used to separate chemical mixtures. I was looking for a specific product of a nucleophilic substitution reaction between a dianhydride and a diamine. When chemists run the products of a reaction through a silica column, molecules exit the column at different times, depending on their polarity, allowing for the isolation of the desired products. Because my reaction produced strongly colored compounds, I could tell which test tubes held different reaction products with the naked eye. My work photo was selected and published by C&EN which is the most important science news. I was proud to mention in c&en that this work has been done at NCSU by me.
Macrolactones
Graduate Student/Postdoc Graphics, 1st Place, by Phyo Phyo Zin
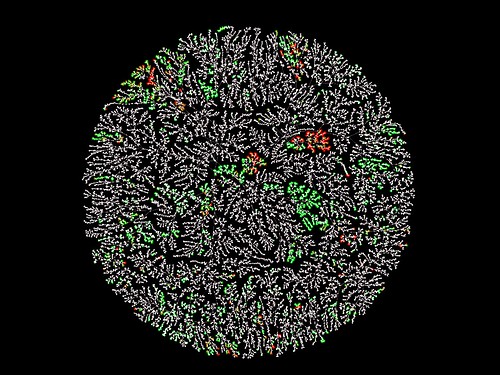
Macrolactones, macrocyclic lactones with at least twelve atoms within the core ring, include diverse natural products such as macrolides with potent bioactivities (e.g. antibiotics) and useful drug-like characteristics. We have developed MacrolactoneDB, which integrates nearly 14,000 existing macrolactones and their bioactivity information from different public databases. The chemical network shows the entire MacrolactoneDB wherein compounds with similar chemical structures and properties are clustered together. Each node is a chemical structure and is colored by its maximal biological activity in the general spectrum (pChEMBL value) from green (high) to red (low). The rest of the chemical space which does not have activities reported in the ChEMBL database is colored white. This technique allows us to visualize the chemical linkages and offers an informative look at underexplored structural classes like macrolactones.
Crystallization Pattern
Graduate Student/Postdoc Photography, Honorable Mention, by Alejandro Valdes, Bram Frohock, Carlos Peniche
Synoxazolidinone derivatives are promising antibacterial compounds with plenty of potential applications in human health yet to be explored. In the Pierce lab, synthetic methods for accessing this kind of natural compound have been developed, and, by making changes in the chemicals used, completely novel synoxazolidinone derivatives with improved biological activity can be prepared. When particularly pure, some analogues precipitate forming beautiful crystalline structures. As if working on the synthesis of promising future antibiotic derivatives were not exciting enough, from time to time, daily lab work delights us with some unusual forms and shapes. Shown in this picture is an intermediate product that was just collected from a purification procedure and concentrated into a round-bottom flask forming a one-of-a-kind, breathtaking crystallization pattern. While working towards making synoxazolidinone antibiotics, these crystal patterns are one of the many pleasant rewards of the lab.