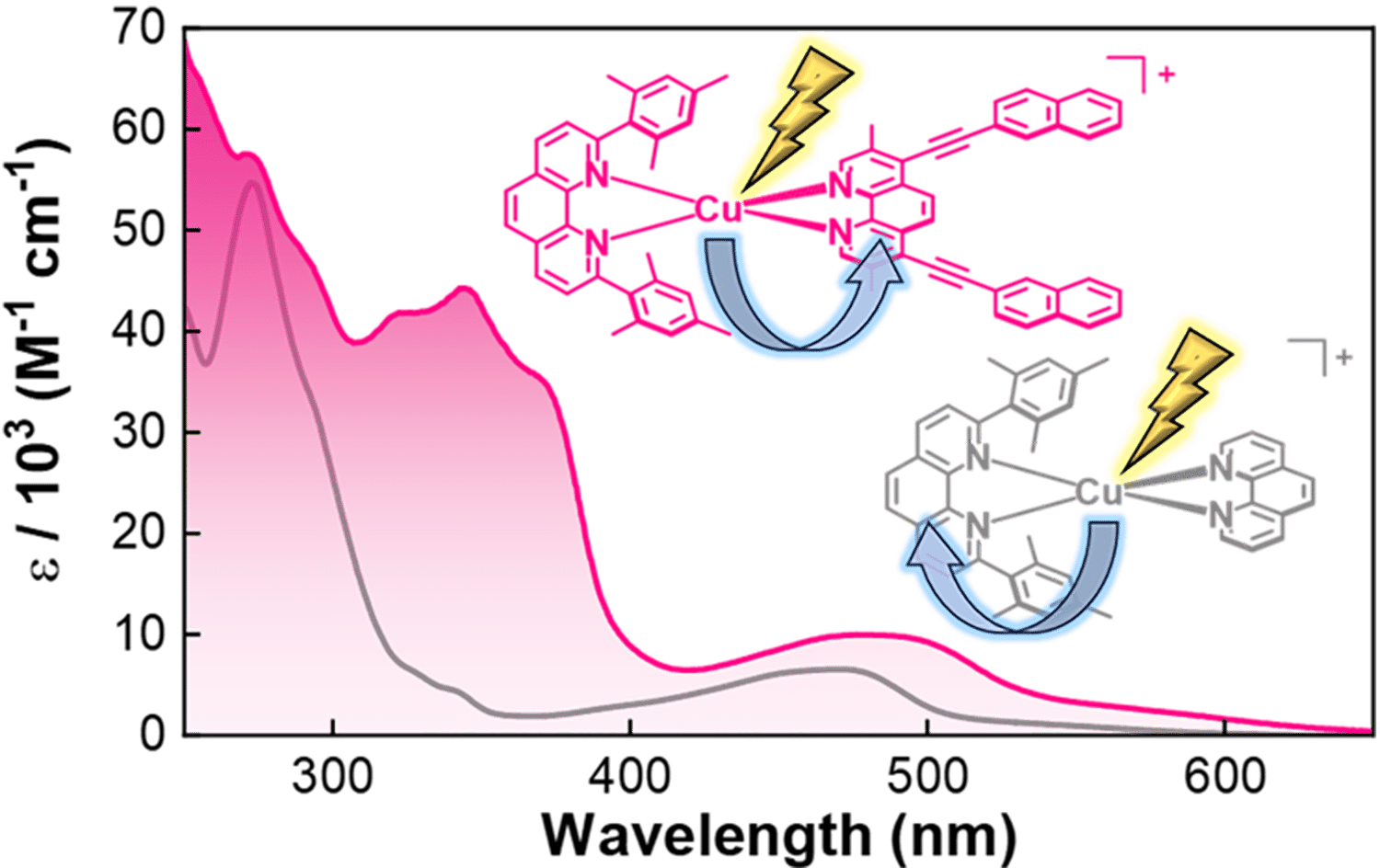
Teaching an Old Reagent New Tricks

Did you know that an old reaction can sometimes be the basis for a new research contribution? A very recent article in the journal Organometallics demonstrated this notion; to specify, the paper is titled “Teaching an Old Reagent New Tricks: Synthesis, Unusual Reactivity, and Solution Dynamics of Borohydride Grignard Compounds” by Allendorf*, Stavila* and coworkers. And the reagent is one that Professor Emeritus Daniel Comins, and a graduate student, Jim Herrick, developed back in 1984.
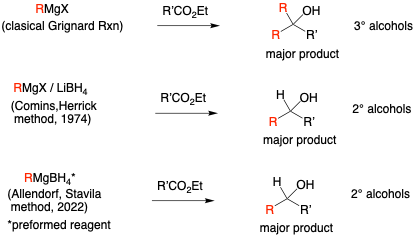
In the present paper, the authors state: “The tandem reactivity (i.e., multiple reactions in one step) and utility of a simple borohydride/Grignard mixture was demonstrated by Comins et al., who reacted standard RMgX reagents in various ratios with LiBH4. The reaction of these reagents with an ester substrate provided secondary alcohols as the major product instead of the tertiary alcohols observed using solely the Grignard reagent.27 Secondary alcohols are desirable both as an intermediate synthon, as well as a chiral precursor for pharmaceuticals and asymmetric catalysis.28 The advantage of an alkyl magnesium borohydride would be a single reagent that can be used in Grignard reactions while also providing a reducing hydride source.”
The doi for the paper by Comins and Herrick is: https://doi.org/10.3390/molecules27061833.